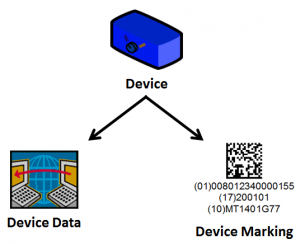
Unique Device Identifier (UDI)
Medical Device Suppliers need to adhere to the FDA UDI Mandate which requires a Unique Device Identifier (UDI) to be assigned and placed on each medical device. The UDI requirement incorporates traceability functionality to improve patient safety and improve supply chain. To assist companies who need to comply with the FDA UDI Mandate, Bar Code Graphics offers;
Educational Resources
- What is the FDA UDI Mandate? UDI
- Implementation schedule for different classes of medical devices. UDI Implementation Schedule
Solutions
- UDI Certification to confirm compliance. UDI Certification
- Printed UDI Labels to apply a pressure sensitive sticker to medical device. UDI Labels
- UDI Software Package for to print and manage UDI barcodes. Software
- Barcode Scanners to support applications which require UDI barcodes. Barcode Scanners
- Mobile Computers with integrate barcode scanners to enable UDI data to be collected and processed in the field. Mobile Computers