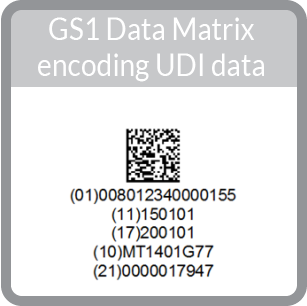
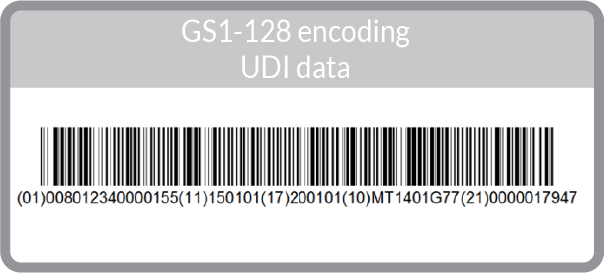
FDA UDI & GS1
To meet FDA UDI (Unique Device Identifier) barcode format requirements, companies may follow one of three standard systems: GS1, HIBCC, and ICCBBA.

The GS1 identification has emerged as the primary system, thanks largely to its already global reach and adoption in several overlapping markets. Their standards cover the formatting, dimensions, and print quality for barcode symbols carrying UDI data, as well as the numbering system used to uniquely identify devices and the data formatting for relevant data fields.
One of the most common GS1-related barcodes is the UPC. This is used to identify retail products throughout the world. It is already in use on many medical devices that are sold in a retail setting.
The two other common GS1 barcodes used in UDI are the GS1-128 linear barcode and the GS1 Data Matrix 2D barcode (Learn more about linear vs 2D here):